
Doing a Systematic Review in Health Sciences
[La revisión sistemática en las ciencias de la salud]
Berta Cajal1, Rafael Jiménez1, 2, Elena Gervilla1, 2, Juan J. Montaño1, and 2
1University of the Balearic Islands, Spain; 2Balearic Islands Health Research Institute (IdISBa), Spain
https://doi.org/10.5093/clysa2020a15
Received 6 April 2020, Accepted 24 April 2020
Abstract
This paper aims to provide a practical, summarized, and clear guide of steps to carry out a systematic review and is aimed at researchers in the field of Health Sciences. The review process runs from the initial questioning to the final report, providing useful information on tools available at each stage. Systematic review and meta-analysis are currently the evidence synthesis tools of the highest level of scientific quality. They are in themselves a secondary research methodology, whose objective is to locate, evaluate, and synthesize the best evidence by selecting original papers or quality primary publications. The procedure to achieve the objective is presented as a sequential and systematized process, in stages, following the transparency principle, so as to ensure its replicability.
Resumen
Este trabajo pretende proporcionar una guía práctica, resumida y clara de los pasos para llevar a cabo una revisión sistemática y está dirigido a los investigadores del ámbito de las ciencias de la salud. El proceso de revisión se desarrolla desde el planteamiento inicial de la pregunta hasta la elaboración del informe final, proporcionando información útil sobre herramientas disponibles en cada etapa. La revisión sistemática y el metaanálisis son actualmente las herramientas de síntesis de evidencia de más alto nivel de calidad científica. Constituyen en sí mismas una metodología de investigación secundaria, cuyo objetivo es localizar, valorar y sintetizar la mejor evidencia seleccionando los trabajos originales o publicaciones primarias de calidad. El procedimiento para alcanzar el objetivo se plantea como un proceso secuencial y sistematizado, por etapas, siguiendo el principio de transparencia, de modo que se asegure su replicabilidad.
Palabras clave
RevisiĂłn sistemática, InvestigaciĂłn, Evidencia, GuĂa, Metaanálisis, Ciencias de la saludKeywords
Systematic review, Research, Evidence, Guide, Meta-analysis, Health sciencesCite this article as: Cajal, B., Jiménez, R., Gervilla, E., & Montaño, J. J. (2020). Doing a Systematic Review in Health Sciences. ClĂnica y Salud, 31(2), 77 - 83. https://doi.org/10.5093/clysa2020a15
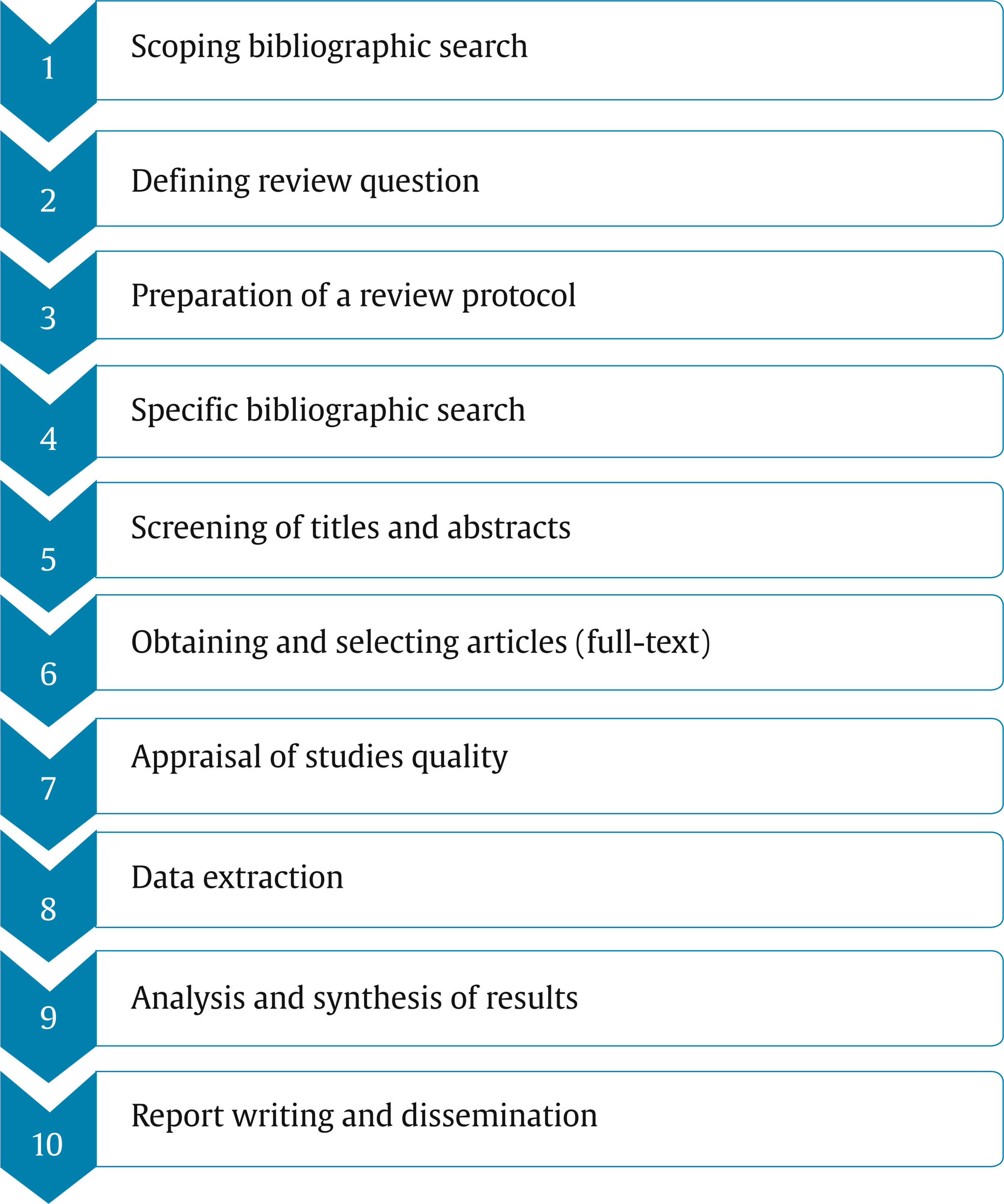
rafa.jimenez@uib.es Correspondence: rafa.jimenez@uib.es (R. Jiménez)In Health Sciences, as in all scientific disciplines, the way of approaching a particular topic of study has completely changed in a few decades due to the high production of publications and the speed at which information is shared through internet (Siddaway et al., 2019). Currently, the volume of publications is growing exponentially, which makes it extremely difficult for researchers and professionals to update their knowledge through direct reading of original studies. It is necessary to optimize the time dedicated to this task by accessing research that brings together a synthesis of highest scientific quality publications and that provide more evidence regarding a specific scientific question. To this it must be added the fact that new research and evidence are constantly emerging with divergent results and conclusions regarding the same question, which support different theories or positions. It could be the conequence of the effect of various factors fundamentally linked to the methodological research process, factors that directly influence the validity of the results obtained and the level of evidence provided. Therefore, it is necessary to carry out a review of the different stages of the research process of different studies, under the same criteria of scientific quality, in order to filter and select studies that provide us with reliable scientific information. Systematic reviews and meta-analyses emerge as the solution to the problem. Both are considered a particular type of publication aimed at providing a comprehensive, rigorous, and inclusive vision of a specific question, providing a synthesis of evidence of the highest level of scientific quality. It is worth differentiating between a qualitative systematic review that puts its efforts into the exhaustive identification of all the literature on a given topic, evaluating its quality and synthesizing its results qualitatively, and a quantitative systematic review whose maximum exponent, meta-analysis, incorporates a specific statistical strategy to synthesize results in a single review. Consequently, we want to note that meta-analysis is not a required element of a systematic review. Brown and Richardson (2017) indicate that “Meta-analysis should only be carried out as part of the review process if data from included intervention studies are sufficiently similar and it is sensible to combine them” (p. 132). There is enough scientific literature available about how to do a meta-analysis if these conditions are met, and we want to suggest the studies by Borenstein et al. (2009), and Botella and Sánchez-Meca (2015). Systematic reviews are considered the best tool for synthesizing scientific evidence. Increasingly, they have become a way to optimize the search for quality information for researchers and a valuable aid for decision-making based on evidence from professionals in the applied field of Health Sciences. Systematic reviews are in themselves a formal method of research (secondary research) based on primary studies. As mentioned, its purpose is to facilitate the understanding of a particular topic by summarizing all the existing evidence in the original studies using a scientific methodology. Although the idea is simple, the execution of the different stages is not always easy, which is why it requires careful and systematic planning through a protocol that must be developed and approved by all authors. It will establish procedures and criteria that will guide the process (Gough et al., 2017). Currently, in Health Sciences there are several international research centres and groups dedicated to conducting systematic reviews, managing, publishing, and storing them, such as Cochrane Collaboration, Campbell Collaboration, PRISMA [Preferred Reported Items for Systematic Revision Reviews and Meta-Analyses], and NHS Centre for Reviews and Dissemination (University of York). Their aim is to promote the methodological training of Health Sciences researchers concerning the synthesis of evidence, and so they offer an extensive arsenal of useful tools and resources on their respective websites (as review tools, online training courses, and webinars). This paper aims to provide a structured and summarized practical guide for Health Sciences researchers considering carrying out a systematic review in order to facilitate the process and minimize the risk of bias in its preparation. Stages of the Systematic Review Process The main principle that should guide the performance of a systematic quality review is transparency in all the procedures that will be developed throughout the entire review process. That is why a priori structured preparation of all stages is needed. The final report should reflect all the information from these steps in a detailed and systematic way to make the review replicable. Additionally, providing information for each stage also allows potential readers or recipients of the publication to assess the quality of findings. Carrying out a systematic quantitative review or a meta-analysis involves covering a series of procedures or tasks that we can structure in ten stages that we will gradually develop (see Figure 1). It is recommended that the review team consists of a minimum of two people who will carry out the entire literature review process. Therefore, they should have the necessary knowledge to evaluate the quality of the studies through risk of bias assessment. It is also essential to have a methodology expert throughout the process, either as a team member or as an external advisor. Scoping Bibliographic Search In order to prepare the systematic review in the area of interest, it is advisable to carry out a first wide-ranging bibliographic search using all the available electronic resources and using generic keywords, in order to know the type of research published in this area. In parallel, it is recommended to extend this initial search to the libraries of the different systematic review centres in Health Sciences already mentioned (such as the NHS Centre for Reviews and Dissemination, Cochrane Collaboration, and Campbell Collaboration), in order to verify that no duplication occurs with the performance of systematic reviews already registered, and thus avoid waste of resources and time. Bibliographic resources that we specifically need in this first stage are the same that we will use in the fourth stage to carry out the specific bibliographic searching; we detail them in section 4; in particular, the use of keywords that belongs to the thesaurus (controlled and structured vocabulary) of consulted databases is highly recommended to obtain more precise results; we expose there this specific resource and recommendations of searching by controlled vocabulary or by free terms. In any case, the query made and publications found will provide information that would allow us to define, in a more precise and appropriate way, the research/review question and, at the same time, will indicate the level of relevance of the problem we are considering. Defining the Review Question The information obtained in the initial bibliographic search will help us to clarify the research/review question more clearly since it will guide subsequent bibliographic search. Its correct definition is crucial in order to optimize search time and ensure results. The next step will consist of expressing the research question in PICO format, the most widely used in the field of Health Sciences (Davies, 2011) and that identifies the necessary elements for subsequent bibliographic search. Its structure has four main components: participants (P), intervention (I), comparison (C), and outcome (O), which allow focusing on relevant aspects of the research question, to which a fifth component can be added referring to the type of study best suited to answer the question asked (S) (PICOS) (see Table 1). It is possible to add a sixth component corresponding to setting (S) (PICOSS) in those cases where it is required. It is required to specify the question, defining the characteristics of participants (e.g., female patients, between 12 and 19 years of age with a diagnosis of DSM-V bulimia nervosa), the intervention or treatment to be tested (e.g., family-based therapy), and the alternative to comparison intervention (e.g., supportive psychotherapy). Regarding the definition of the outcome variables, if necessary, primary outcome variables should be differentiated. Those that will be analysed to draw main conclusions (e.g., abstinence from binge-eating and purging episodes, measured through Eating Disorder Examination) and secondary for subgroup analysis/comparison (e.g., frequency of binge and purge episodes measured through Eating Disorder Examination and total scores). When defining the type of study to be selected, it is advisable to consider the adequacy of the design to the set revision question (see Table 2). Once the review question has been established, it is necessary to define and specify inclusion and exclusion criteria. Inclusion criteria are those specific characteristics that the study must meet to be included in the review; they are the eligibility criteria. Exclusion criteria are characteristics that a study must have to be automatically excluded. Often, definition of the problem and definition of inclusion/exclusion criteria are developed in parallel since they are complementary tasks. By defining the review question, we are, at the same time, establishing specific inclusion and exclusion criteria. Some of these specified criteria may refer to characteristics of the publication. As an example, we will cite the most commonly used, such as language, date of publication, study design, sample size/number of subjects included, outcome variables, inclusion of comparison group, population, study unit, and characteristics of the intervention. In order to avoid introducing biases in selection through inclusion/exclusion criteria, it is recommended that special attention be paid to language bias and publication bias. On the same topic, studies that have obtained positive results are more likely to be accepted and published in peer-reviewed journals and in English, while papers with negative results tend not to be accepted and are published in non-English journals or, directly, they are not published (grey literature). It is essential to consider this aspect to assess the type of evidence that is desired through selection. Preparation of a Review Protocol At this point, a protocol should be drawn up where structured planning of the entire process to follow to carry out the work will be reflected. It will be the road map of the review, thus avoiding biases along the way and ensuring transparency of the review process. This protocol must be prepared and agreed by all members of the review team, and its compliance must be respected. Some organizations or systematic review centres in Health Sciences such as Cochrane, Campbell Collaboration, or the Agency for Healthcare Research and Quality (AHRQ) require their use and offer protocol templates, although not always in open access. However, templates have rarely been published in traditional journals, which has meant that systematic reviews in Health Sciences have been carried out for years that in no case mention having followed a protocol (Shamseer et al., 2015). Intending to improve this aspect, the PRISMA-P group prepared a list of 17 items structured in a checklist that, at the same time, are a guide to prepare the review report. Furthermore, in order to promote the use of these protocols, PROSPERO, a portal for prospective registration of systematic review protocols before they are carried out, helps avoid duplication (Moher et al., 2015). Even though there is no standard template or an explicit agreement between international Health Sciences review centres or expert groups regarding the specific format or what attributes a protocol template should collect, they are all coincident and share a basic structure (see Table 3). Specific Bibliographic Search In this stage, we will use again bibliographic resources (in a more accurate way than in stage 1) to address the review question. We will also need resources to carry out organization and management of evidence we have found (i.e., bibliographic management software). Consequently, to address this kind of resources we can classify them in two categories (A/B): A. Using platforms or providers of documental and bibliographic information. The following steps are essential to ensure proper use of these resources: Step 1. Planning your main search: the main search should be adequately balanced in terms of specificity (you should identify enough relevant evidence) and sensitivity (you should not identify too many irrelevant sources of evidence). Furthermore, to ensure this specificity and reduce the effect of publication bias, it is important to consider the two sources of evidence that are available – published literature (controlled by commercial publishers) and grey literature (not controlled by commercial publishers). In this sense, Dundar and Fleeman (2017) state that “it is a common misconception that systematic reviews should only include published literature” (p. 65). Consequently, grey literature searches can identify ongoing or unpublished studies, often not limited by restricted word count, and probably a larger number of studies showing a null or negative effect can be found. Also, grey literature can be a good source for very recent research results, such as conference proceedings. Therefore, planning main search with published and grey literature will help to design search strategies. Step 2. Search strategies:
Step 3. It would be convenient to be aware of the visibility level (impact) of selected references. Consequently, we search for quality indicators of journals that have published our target references, how many citations accumulate a reference or work, and other indicators collected in PlumX Metrics database. We detail these indicators:
B. Using bibliographic software to reference management. In the review process, organized storage of information is essential, and, it is very advantageous to handle some bibliographic software package to facilitate storage and use of the collected references (e.g., Endnote, RefWorks, Mendeley). A bibliographic software offers the following benefits: automatic download (web-importing process) of selected references from bibliographic databases by descriptors fields (titles, abstracts, keywords, etc.); electronic storage of all reference information, including notes, images, and full-text pdf files; ability to group and organize references; adding and formatting in-text citations and bibliographies in multiple styles (e.g. APA or Vancouver referencing style) using a word processor plugin; and the ability to share your references. It should be noticed that bibliographic searching is a critical part of the systematic review and a mistake in this process involves obtaining incomplete searches and, therefore, biased evidence on which the review will be based. For this reason, in case of not having the needed specific knowledge about information retrieval, it is advisable to count with a searching expert in the team. Screening of Titles and Abstracts Bibliographic search is likely to provide a large number of results that must be exported to a bibliographic manager (resource defined in section 4). At this stage, the aim is to filter this accumulation of references. Through a bibliographic manager, first filtering can be carried out since it allows identifying and deleting duplicate references from the same work. The second filtering will consist of reading titles and abstracts of all stored references. A large number of results will be irrelevant and easily disposable. With the rest, it is time to apply inclusion and exclusion criteria concerning the relation to PICO parameters, and for this it is convenient to create a filtering table or checklist (e.g., using Access or Excel) where compliance or not of each of them can be written down for each reference. Screening tasks at all stages should be carried out in parallel by at least two reviewers, independently, which will reduce selection bias. In this first screening stage, in case of doubt, it is advisable to be conservative and not discarding the article. In case of any discrepancy between reviewers regarding the selection or not of an article, it should be resolved following the criteria established in the protocol for these cases, usually through discussion and consensus in the application of inclusion/exclusion criteria. In case of not reaching an agreement, their eligibility will be evaluated later, through full-text reading. The final report should include the degree of agreement between reviewers (e.g., through kappa index). When the goal is to carry out a meta-analysis, it will be convenient to verify the effect of its inclusion or exclusion in the result set through sensitivity analysis (e.g., Forrest plot) (Molina, 2018; Needleman, 2002). Obtaining and Selecting Articles After the first screening and once papers considered potentially eligible have been identified, it is essential to have the full-text version of all the articles, published and unpublished. In this second screening, when reading the papers, inclusion and exclusion criteria are applied again, strictly. The filtering table created in the previous step will be used again, and reasons for inclusion and exclusion of each article will be detailed. This stage should also be carried out by two separate persons, so in the event that there is no consensus among them a third independent reviewer will be consulted. It is recommended that these discrepancies between reviewers and their resolution be evidenced in an incident register. The entire selection process of the resulting set of articles that will be used to carry out the systematic review must be reflected in a flow chart, where the screening process carried out will be detailed step by step (Liberati et al., 2009; Moher et al., 2009). PRISMA group (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) makes an editable flow chart template structured in four phases available (http://prisma-statement.org/PRISMAStatement/FlowDiagram). Appraisal of Studies Quality Works finally selected through the filter of inclusion and exclusion criteria, may vary regarding their quality. Assessing the quality of a research means evaluating the methodological quality of the study, that is, the rigour applied to minimize bias and error in the design, rigour in its conduct, and analysis of results (Boland et al., 2017). According to recommendations of Cochrane Collaboration guidelines and PRISMA Statement, a series of characteristics grouped into five factors should be evaluated: selection bias, performance bias, detection bias, attrition bias, and reporting bias (Higgins et al., 2019; Liberati et al., 2009; Perestelo-Pérez, 2015). Evaluating the five factors involves examining design characteristics such as sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, and percentage of dropped out, among others (Berkman et al., 2014). Even though these characteristics are directly selected from experimental studies, authors identify their usefulness for other types of studies. In Health Sciences, there is currently a wide range of tools and differentiated checklists, adapted to assess the validity of each type of study, whether experimental, quasi-experimental, ex-post-facto, qualitative, for example. They differ in terms of validity, psychometric properties (the scales allow obtaining a score as a quality assessment and the checklists provide an overall assessment) and the scope of the methodological review (Armijo-Olivo, et al., 2008; Jarde et al. 2012; Zeng et al., 2015). Below we highlight the most prominent and commonly used, differentiating them by type of study:
Both SIGN and CASPe have templates for all types of studies. A highly recommended resource is Temple University Libraries. On its website it has a very comprehensive catalogue of critical appraisal checklists classified by specific study design type. The website also gives access to high standards variety of systematic reviews assessment tools. In any case, the correct identification of the type of study is the priority to select the right tool to carry out the evaluation. Again, at this stage, assessment of each work must be done by at least two independent reviewers. The results of the evaluation of each article and for each valued item will be presented together in a summary table. In case of performing a meta-analysis, the scores obtained in the evaluation scales can be used, as weights or, instead, a sensitivity analysis. Data Extraction After assessing the quality of papers and once identified the studies to be included in the review, the next step will be to identify and extract relevant data from each article for subsequent analysis and synthesis. This information should be collected in a single format (through a data extraction table or an extraction template). Data to be extracted are not the same for all systematic reviews since they depend on the research question and the topic, and therefore, data collected will be related to inclusion/exclusion criteria. However, the information that is recorded usually includes the following basic aspects: authors (first author), publication type, citation, year of publication, key measures or variables, study period, research design (aspects that have to do with the risk of bias such as blinding, concealment,...), number and characteristics of participants (including any dropout), description of intervention and comparison, outcome measures (primary outcome and secondary outcome, if needed), study settings, number of participants included in the study, length of follow-up up, conclusions, study sponsorship, etc. The demographic information of study’s participants must also be extracted, which will vary depending on the research question but must at least include participants’ age and gender. In specific reviews, it will be necessary to extract more specific characteristics, such as ethnicity, educational level, socio-economic level, or specific physical or mental health diagnoses. In the case of randomized designs (such as randomized control trials), it will be necessary to extract all the baseline information of participants at this stage. In general, it is advisable to extract the maximum amount of information in this phase to avoid having to revisit the study in case lack of relevant data is subsequently detected, which can be time and energy-consuming (Perestelo-Pérez, 2013; Siddaway et al., 2019). Publications that do not meet inclusion criteria and are rejected must be reflected in the corresponding extraction table, mentioning the reason for their exclusion: blinding or lack of randomization, for example. Again, in this phase it is recommended that data extraction is carried out by at least two independent reviewers and that in case of discrepancies a third reviewer resolve disagreements. Cochrane Collaboration uses a new standard platform for all its publications, Covidence, to which Campbell Collaboration has joined, in order to produce systematic online reviews in Health Sciences, Social Sciences, and Education, so that all data extraction is entered directly through this platform that allows importing and exporting information to a bibliographic manager (RevMan) and Excel, as well as obtaining tables and evidence maps. Analysis and Synthesis of Results In next step, the aim is to combine, integrate, and synthesize the evidence extracted from the different works reviewed in order to answer the review question asked about the effectiveness of an intervention (defined in PICOS parameters). This question means asking for: a) what is the general effect of the intervention?, b) are there differences in the effect of the intervention between different studies?, and c) in the case of different effects between studies, what are the factors that could affect this heterogeneity of the results? (Sánchez-Meca & Botella, 2010). When the different studies included in the systematic review are very heterogeneous because they present differences, for example, in terms of characteristics of subjects, aspects of the design used, application of interventions, or different outcome variables, or different quality of studies, combination of quantitative results should be carried out qualitatively through a narrative synthesis. For this, a combined table will be structured in which results (according to type of data) of each intervention will be presented, together with a summary statistic or point estimate of direction and size of treatment effect. A qualitative assessment will be made of data provided. Only when studies are sufficiently similar, that is, homogeneous, can a meta-analysis be carried out. Meta-analysis is a statistical method that allows results of different studies to be combined and provide a single estimate of higher statistical power for the measurement of the intervention effect. As we have already pointed out, meta-analysis is not a requirement in a systematic review, it can be part of the review if certain assumptions regarding homogeneity are met: certain aspects of subjects must be similar (characteristics linked to criteria of inclusion and baseline, fundamental aspect when studies are quasi-experimental); the same interventions should be compared with same comparators; same outcome variables must be recorded and during the same time usage; and finally, all studies must describe similar effects (it can be visualized through a Forrest plot). However, when studies included do not show similar results regarding the size of the treatment effect, the meta-analysis report should include the application of a heterogeneity test in order to find out if the diversity of results is due to factors underlying or random (Higgins et al., 2003). In any case, the decision on which strategy to follow in the evidence synthesis stage will be totally determined by the type of studies included in the systematic review. Given the transparency of the whole process, a qualitative review could only introduce biases when drawing conclusions, if more attention is paid to specific study results. If requirements are met, a meta-analysis will always be preferable to a qualitative review. At this stage, it is highly recommended to count on a methodologist or statistician to check the adequacy of performing or not a meta-analysis according to available data. A methodologist will provide advice in order to identify the best method to apply to synthesize evidence, the appropriate software to use in each case, and useful information about understanding the results. Report Writing and Dissemination The writing of the final report constitutes the last step in the process of preparing the systematic review, together with its subsequent dissemination. It is important that the report has quality standards, which means transmitting the transparency of the entire review process in order to guarantee its replicability. In Psychology, APA designed MARS guide [Meta-Analysis Reporting Standards] (APA Publications and Communications Board Working Group on Journal Article Reporting Standards, 2008) inspired by PRISMA, QUORUM, and MOOSE guides, with the double utility to guide researchers in preparing quantitative systematic reviews (meta-analysis), and in assessing their quality (Appelbaum et al. 2018; Rubio-Aparicio et al., 2018) but it is, at the same time, a guide of elements that should be included when writing the report of the systematic review. MARS guide consists of 74 items structured in: title, abstract, introduction, method, results, and discussion. The use of quality assessment guidelines allows detecting weak points of the process carried out before writing conclusions. It can be beneficial when the review must be sent for publication, to ensure the accomplishment of quality standards. In addition to being tools for evaluating the quality of the systematic review, they are in themselves a standardized guide to carry them out, and some of them include certain aspects of the process, such as construction of a protocol and the study of bias and heterogeneity. In Health Sciences, the most frequently used are:
As we have already seen, conducting a systematic review is a hard-working and lengthy process, and writing the final report is not easy. It is important that the report collects all the information on each of the decisions made throughout the process as a measure of transparency and proof of its quality. It should not be forgotten that the conclusions of this type of secondary synthesis research in Health Sciences may have a very significant impact in all areas (policy setting, applied healthcare, and research, for example), given that they are located at the top of the hierarchy of quality levels of evidence provided. Conflict of Interest The authors of this article declare no conflict of interest. Acknowledgement We want to express our gratitude to Prof. Albert Sesé, for his wise advices in carrying out this work. Cite this article as: Cajal, B., Jiménez, R., Gervilla, E., & Montaño, J. J. (2020). Doing a systematic review in health sciences. Clínica y Salud, 31(2), 77-83. https://doi.org/10.5093/clysa2020a15 References |
Cite this article as: Cajal, B., Jiménez, R., Gervilla, E., & Montaño, J. J. (2020). Doing a Systematic Review in Health Sciences. ClĂnica y Salud, 31(2), 77 - 83. https://doi.org/10.5093/clysa2020a15
rafa.jimenez@uib.es Correspondence: rafa.jimenez@uib.es (R. Jiménez)Copyright © 2026. Colegio Oficial de la Psicología de Madrid












 e-PUB
e-PUB CrossRef
CrossRef JATS
JATS







