
Neuropsychology and Quantitative Electroencephalography in a Case of Frontotemporal Dementia and Small Vessel Disease
[Neuropsicolog├şa y electroencefalograf├şa cuantitativa en un caso de demencia frontotemporal y enfermedad cerebral de peque├▒os vasos]
Adrián Galiana1, 2, Ana I. Campos-Varillas2, Melanie Blasco-González2, and María Vela-Romero2
1Universidad a Distancia de Madrid (UDIMA), Collado Villalba, Madrid, Espa├▒a; 2Conecta Cl├şnica, Ciudad Real, Espa├▒a
https://doi.org/10.5093/clysa2024a2
Received 1 April 2023, Accepted 4 October 2023
Abstract
Neuropsychological assessment is essential in patients with cognitive impairment and could be coupled with psychophysiological techniques. Specifically, quantitative electroencephalography may complement data from neuroimage, neurophysiology, and neuropsychology providing relevant information about brain functioning and dynamics. A 77-year-old male consulted for expressive language difficulties that began at age 65. Neuroimaging findings revealed atrophy and hypometabolism of the left temporal and frontal lobe, with cerebral microlesions. Quantitative electroencephalography findings showed decreased absolute and relative power, low alpha peak frequency, and marked inter- and intrahemispheric frontal and temporal asymmetries. The neuropsychological profile showed alteration in executive and expressive language domains, consistent with neuroimaging and psychophysiological findings. A diagnosis of primary progressive aphasia, a form of frontotemporal dementia, as well as a small vessel disease and mild cognitive impairment was concluded. Neuropsychological and quantitative electroencephalography data contribute to the diagnosis and would help determine the disease progression.
Resumen
La evaluación neuropsicológica es fundamental en pacientes con deterioro cognitivo y podría complementarse con técnicas psicofisiológicas. Específicamente, la electroencefalografía cuantitativa puede complementar datos de neuroimagen, neurofisiología y neuropsicología, proporcionando información relevante sobre el funcionamiento y la dinámica del cerebro. Varón de 77 años que consulta por dificultades en el lenguaje expresivo que comenzaron a los 65 años. Los hallazgos de neuroimagen revelan atrofia e hipometabolismo del lóbulo temporal y frontal izquierdo, con microlesiones cerebrales. Los hallazgos de la de electroencefalografía cuantitativa muestran una disminución de la potencia absoluta y relativa, una frecuencia de pico alfa baja y marcadas asimetrías frontales y temporales inter e intrahemisféricas. El perfil neuropsicológico muestra alteración en los dominios ejecutivo y del lenguaje expresivo congruente con los hallazgos de neuroimagen y psicofisiológicos. Se concluye diagnóstico de afasia primaria progresiva, una forma de demencia frontotemporal, así como enfermedad cerebral de pequeños vasos y deterioro cognitivo leve. Los datos neuropsicológicos y electroencefalográficos cuantitativos contribuyen al diagnóstico y podrían ayudar a determinar la progresión de la enfermedad.
Palabras clave
Neuropsicolog├şa, Electroencefalograf├şa cuantitativa, Demencia frontotemporal, Enfermedad cerebral de peque├▒os vasos, Neuroimagen, Psicofisiolog├şa, AfasiaKeywords
Neuropsychology, Quantitative electroencephalography, Frontotemporal dementia, Small vessel disease, Neuroimage, Psychophysiology, AphasiaCite this article as: Galiana, A., Campos-Varillas, A. I., Blasco-González, M., & Vela-Romero, M. (2024). Neuropsychology and Quantitative Electroencephalography in a Case of Frontotemporal Dementia and Small Vessel Disease. Cl├şnica y Salud, 35(3), 95 - 99. https://doi.org/10.5093/clysa2024a2
Correspondence: adrian.galiana@udima.es (A. Galiana).Frontotemporal atrophy comprises the degeneration of frontal and temporal lobes, due to neuronal loss associated with different pathophysiological processes (Tartaglia & Mackenzie, 2022). The cerebral small vessel disease is mainly characterized by the presence of cerebral microinfarcts, white matter chronic ischemia, or cerebral microhemorrhage (Elahi et al., 2023). Patients with both frontotemporal atrophy and small vessel disease present cognitive symptoms that may progress to dementia. In this context, the neuropsychological assessment is essential to determine the severity of cognitive symptoms and their emotional and behavioral impact. In addition to psychometric instruments, clinical and research practice in neuropsychology includes objective or psychophysiological assessment tools. These include quantitative electroencephalography (qEEG), which is commonly used in the study of neurodevelopmental and neurocognitive disorders among psychophysiologists (Galiana-Simal et al., 2020). A qEEG is a computer analysis of the EEG signal for the calculation of quantitative parameters, allowing a more in-depth study of brain functioning and dynamics than traditional EEG. For each frequency range (delta, theta, alpha, beta, and gamma) absolute power, relative power, power ratios, and connectivity measures are generated (Livint Popa et al., 2020). It also allows the application of algorithms for the precise localization of the signal source by Low-Resolution Electromagnetic Tomography (LORETA; Ikeda et al., 2019). All measures can be compared with a normative or clinical reference database to estimate deviations, allowing the association between the neuropsychological profile with objective measures of brain functioning. However, despite its safety, low cost, and objective information provided, its potential remains unknown to many professionals. Figure 1 Neuroimaging and Quantitative Electroencephalography Results.  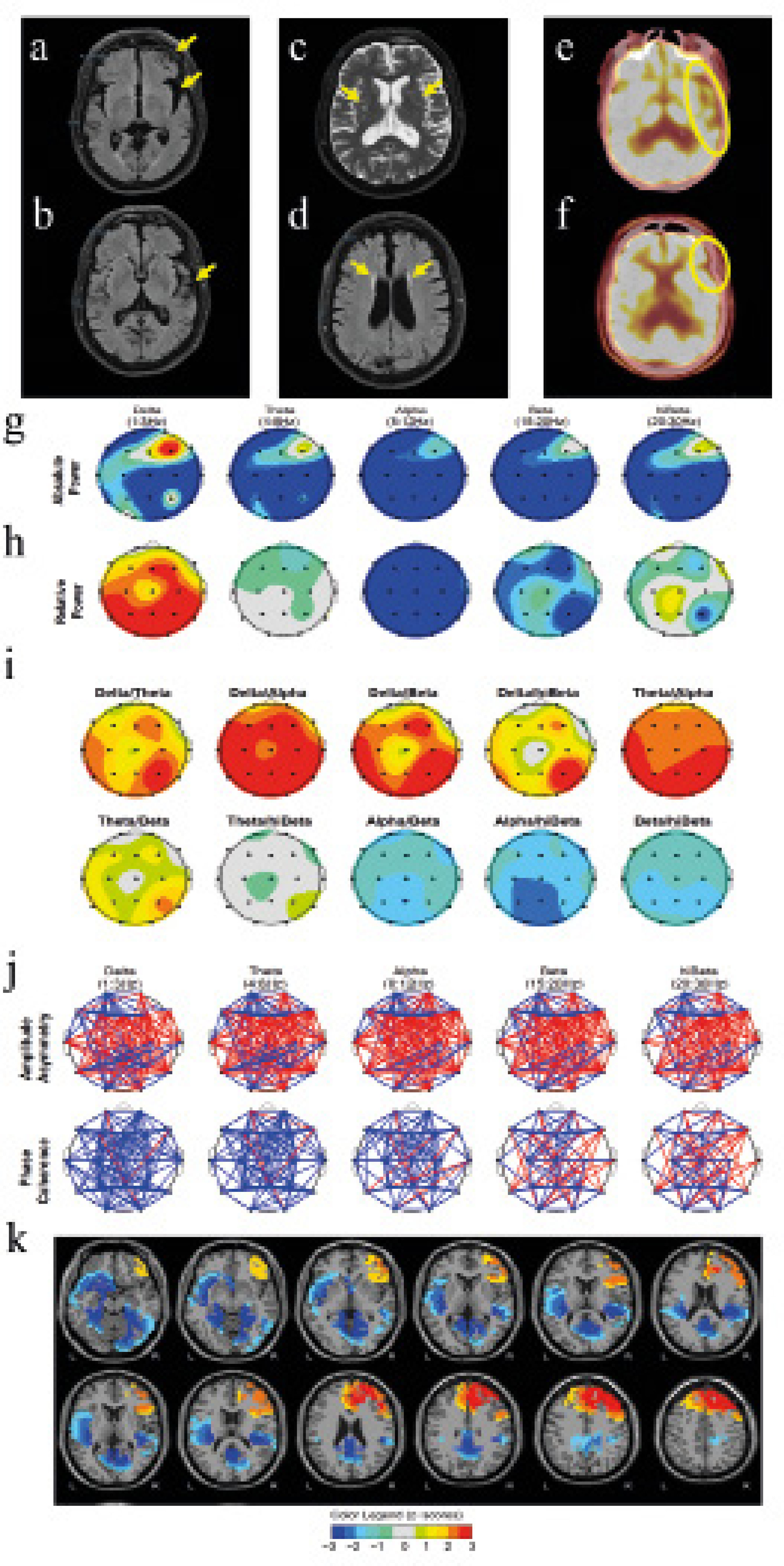 Note. Results of of neuroimaging (a-f) and quantitative electroencephalography (g-k). T2-FLAIR brain MRI (a and b), showing left frontal and left temporal atrophy; T2 brain MRI (c), showing microlesions in bilateral subcortical white matter; T2-FLAIR brain MRI (d), showing lesions in bilateral periventricular white matter; and FDG PET-CT (e and f), showing left frontal and left temporal hypometabolism. The qEEG graphs show, for each frequency range, topographic distribution of absolute signal power (g); relative power (h); ratios between relative powers (i); parameters related to synchronization between areas, such as asymmetry and coherence (j). Finally, images of the LORETA analysis are shown with the location of the extreme signal sources, in different axial slices (k). In the qEEG plots, the colors indicate z-scores (red means patient’s deviations above average when compared to normative database, and blue, deviations below average). The aim of this study is to show the usefulness of qEEG as a complementary tool to neuropsychological and neuroimaging evaluation. Specifically, we show the diagnostic process of a case of frontotemporal dementia and small vessel disease in a patient who visited for difficulties in expressive language after 14 years of slow progression. The associations between neuroimaging and neuropsychological findings with psychophysiological qEEG data are discussed. Case Study A retired businessman aged 77, with basic education, married, with one daughter, and no toxic habits, visited a primary care unit with her wife, reporting behavioral changes, social withdrawal, and depressive symptoms that approximately began at age 65. In addition, his wife indicates language problems, which have worsened over the years, decreasing in spontaneity, frequency, and fluency, that are progressing into monosyllabic communication. The patient’s medical history includes hypertension, type 2 diabetes, and dyslipidemia under pharmacological control and periodic follow-up. There were no family antecedents of dementia or other neurological or psychiatric diseases. Informed consent was obtained for the anonymous publication of clinical data with research objectives. Physical examination of the patient showed no relevant findings. The mental status examination showed evident alteration of the communicative capacity and possible depression. Complementary tests included blood tests, cranial magnetic resonance imaging (MRI), 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET-CT), and neuropsychological evaluation. In addition, EEG activity of 19 cortical positions according to the 10/20 system was recorded under a resting eyes-closed state with linked-ears reference, for subsequent complementary qEEG analysis. Regarding the blood test, it revealed no alterations. The MRI study showed cortico-subcortical atrophy with frontal and temporal predominance (Figure 1a-b) and hyperintensities in white matter of bilateral subcortical and periventricular areas due to microlesions of vascular origin (Figure 1c-d). The FDG PET-CT study showed frontotemporal hypometabolism with marked predominance in the left hemisphere (Figure 1e-f). The metabolic activity of the basal ganglia, thalamus, midbrain, brainstem, and cerebellum showed no alterations. The neuropsychological assessment was performed after an initial semi-structured interview, with the instruments detailed in Table 1. A first cognitive screening round revealed mild to moderate cognitive impairment (Mini-mental state examination 24/35, Montreal cognitive assessment 18/30) with substantial impairment in delayed and working memory, verbal fluency, and sentence repetition. Further examination (Table 1) showed mild impairment in digit forward span, verbal learning ability, semantic and verbal fluency, automatic reading, inhibitory ability, and cognitive flexibility. The task that showed the most severe impairment, with null performance, was the digit backward span. Mild cognitive impairment was concluded with verbal learning impairment and altered language, especially in oral production, fluency, and repetition, with preservation of comprehension, dysarthria, and no paraphasia, compatible with Broca aphasia, as well as partial alteration of executive functioning, with very low performance in working memory and cognitive flexibility. In addition, instruments were administered to explore the presence, frequency, and severity of neuropsychiatric and specific symptoms of anxiety and depression, with results of possible moderate depression and mild anxiety (Table 1). The patient showed mild dependence in basic activities of daily living and instrumental activities and no social risk was detected (Table 1). Regarding the qEEG, the most relevant results were a generalized decreased absolute power, except in the right frontal area, which showed elevated power peaks in the slow and fast frequency waves (Figure 1g) and an average alpha peak frequency shifted at low frequencies (7.23Hz with 0.41 standard deviation), which is considered slow, very high delta relative power, with a generalized decrease in the other frequencies (Figure 1h), high slow-wave to fast-wave ratios (Figure 1i), and very altered synchronization between different brain areas, with marked inter- and intrahemispheric frontal and temporal asymmetries (Figure 1j). Finally, LORETA analysis of extreme deviation values (Figure 1k) showed a marked prefrontal asymmetry signal with excess in the right prefrontal lobe and defect in bilateral parahippocampal gyrus and bilateral posterior cingulate, as well as in left inferior frontal gyrus, left superior temporal gyrus, and left insula. The conclusion was primary progressive aphasia, a form of frontotemporal dementia, as well as small vessel disease, with mild cognitive impairment. Donepezil 10 mg and escitalopram 15 mg were prescribed. Intervention in neuropsychology and speech therapy began with the aim of preserving unimpaired cognitive skills, providing alternative communication systems and for the management of affective symptomatology. We present a case that started with difficulties in the language area, with a personal history of hypertension, diabetes, and dyslipidemia, which, after study, revealed atrophy and hypometabolism of the left temporal and frontal lobes, with microlesions of vascular origin in bilateral subcortical and periventricular white matter. Neuropsychological assessment showed alterations in executive and language domains, with moderate depressive and mild anxious symptomatology. These findings were complemented with a qEEG to establish associations with neuroimaging and neuropsychology. The qEEG data showed close relationship with the rest of the findings. Frontotemporal atrophy has been previously related to low alpha peak frequencies (around 7.5-8.5) and increased relative slow wave contribution, as well as inter- and intrahemispheric asymmetries (Passant et al., 2005), as shown by the patient under analysis. It has also been described that the increase in frontal and temporal slow wave power correlates with PET images showing hypometabolism in these areas and, in a similar way, the qEEG findings in connectivity parameters correlate with the information provided by DTI imaging studies showing damage in white matter (Koberda, 2021); these findings were also common in our case. Particularly relevant, the patient showed excess right prefrontal power, corroborated by LORETA analysis and marked prefrontal asymmetry. This type of findings have been related to depressive states and also to executive dysfunction (Saletu et al., 2010), again, characteristics presented by the patient. In addition, the progressive increase in power and relative contribution of low frequency waves, as well as the shift of the alpha peak to lower frequencies, has been consistently related in the literature to the progression of cognitive impairment to dementia, being particularly relevant parameters when estimating disease progression (Hamilton et al., 2021). Finally, a decrease in the left temporal and frontal signals stand out, which could be associated to the neuroimaging findings and the marked impairment of expressive language. These relationships, although consistent with the findings of the other tests and with the literature, should not, in our opinion, replace the usual diagnostic procedures, but rather complement them. Therefore, the combined use of neuroimaging, neuropsychological assessment, and qEEG may help clinicians to higher diagnostic specificity and grade severity. Although qEEG provides a large amount of objective and specific information about a patient’s brain, a given qEEG found may not be pathology-specific and can be found in different conditions limiting its use, requiring, at least, additional neuropsychological assessment (Salcini et al., 2020). Therefore, we consider that qEEG may find its best utility as a tool in the context of neuropsychological assessment, so that the professional can contrast and associate neuropsychological findings with the underlying brain psychophysiology; however, due to its limitations, diagnosis of structural brain alterations will require neuroimaging methods. Moreover, objective, psychophysiological data from qEEG could be useful in the follow-up of patients and the study of the disease progression or the effect of therapies, both pharmacological and non-pharmacological, so that this information could be associated to observed cognitive, emotional, and behavioral changes. In conclusion, qEEG is an accessible technology that, despite limitations, provides objective and useful brain psychophysiological information for neuropsychological practice. Conflict of Interest The authors of this article declare no conflict of interest. Cite this article as: Galiana, A., . Campos-Varillas, A. I, Blasco-González, M., & Vela-Romero, M. (2023). Neuropsychology and quantitative electroencephalography in a case of frontotemporal dementia and small vessel disease. Clínica y Salud, 35(3), 95-99. https://doi.org/10.5093/clysa2024a2 References |
Cite this article as: Galiana, A., Campos-Varillas, A. I., Blasco-González, M., & Vela-Romero, M. (2024). Neuropsychology and Quantitative Electroencephalography in a Case of Frontotemporal Dementia and Small Vessel Disease. Cl├şnica y Salud, 35(3), 95 - 99. https://doi.org/10.5093/clysa2024a2
Correspondence: adrian.galiana@udima.es (A. Galiana).Copyright © 2026. Colegio Oficial de la Psicología de Madrid









 e-PUB
e-PUB CrossRef
CrossRef JATS
JATS







