
VR Cognitive-based Intervention for Enhancing Cognitive Functions and Well-being in Older Adults with Mild Cognitive Impairment: Behavioral and EEG Evidence
Pattrawadee Makmee1 and Peera Wongupparaj2
1Department of Research and Applied Psychology, Faculty of Education, Burapha University, Thailand; 2Department of Psychology, Faculty of Humanities and Social Sciences, Burapha University, Thailand
https://doi.org/10.5093/pi2025a4
Received 3 May 2024, Accepted 8 November 2024
Abstract
Objective: Mild cognitive impairment (MCI) has been recognized as a window of opportunity for therapeutic and preventive measures to slow cognitive decline. The current study investigated the efficacy of the virtual reality (VR) cognitive-based intervention on verbal and visuospatial short-term memory (STM), executive functions (EFs), and wellbeing among older adults with and without MCI. Method: The immersive VR cognitive-based intervention comprised eight 60-minute sessions, held twice a week over a span of 30 days. The participants consisted of 31 non-MCI older adults in the experimental group (mean age ± SD = 66.31 ± 3.12 years), 29 older adults with MCI in the experimental group (mean age ± SD = 68.19 ± 5.03 years), and 30 non-MCI older adults in the control group (mean age ± SD = 64.97 ± 3.35 years). The dependent variables were assessed by using a battery of computerized test, the well-being of older people questionnaire and resting-state EEG. A repeated-measures ANCOVA was employed to examine the effects of the developed VR intervention. Results: Significant improvements were observed in both STMs and EFs following the intervention, as indicated by behavioral and EEG findings, ranging from small to large effect sizes (i.e., = .05-.17). However, enhanced wellbeing was specifically observed among older adults with MCI in the experimental group, F(2, 87) = 6.78, p .01, = .11. Conclusions: The present findings lend support to the efficacy of VR cognitive-based interventions across clinical and non-clinical populations. These results underscore the immediate impact of the intervention across multimodal assessments, including neurophysiological changes, cognitive, and behavioral outcomes.
Keywords
Cognitive enhancement, VR intervention, Mental health, EEG, Absolute and relative powerCite this article as: Makmee, P. & Wongupparaj, P. (2025). VR Cognitive-based Intervention for Enhancing Cognitive Functions and Well-being in Older Adults with Mild Cognitive Impairment: Behavioral and EEG Evidence. Psychosocial Intervention, 34(1), 37 - 51. https://doi.org/10.5093/pi2025a4
Correspondence: peera.wo@go.buu.ac.th (P. Wongupparaj).With the rapidly growing number of older adults, maintaining functional independence, preserving well-being, and communicating with others in advancing age require cognitive functioning without significant difficulty (Murman, 2015). Nonetheless, it is evident that aging-related physical and cognitive declines affect the performances of older adults across a number of cognitive domains, including processing speed (Bonnechère et al., 2021; Gkotzamanis et al., 2021; Ticha et al., 2023; Verhaeghen, 2013), verbal and visuospatial short-term memory (Bopp & Verhaeghen, 2005; Durteste et al., 2023; Mitchell & Cusack, 2018; Verhaegen & Poncelet, 2015), executive functioning (Ferguson et al., 2021; Idowu & Szameitat, 2023; Rodríguez-Aranda et al., 2016), and also physical and psychological well-being (Górska et al., 2021; Iwano et al., 2022; Mak et al., 2023). Cognitive decline and impairment also increased the risk of mortality among community-dwelling and hospitalized older adults, in parallel with the degree of cognitive impairment (Abess et al., 2023; Ablett et al., 2019; Bae et al., 2018; Campayo et al., 2015). Specifically, compared with cognitively preserved older adults, older adults with cognitive impairment experience an increase in mortality rate around 30% (Lee et al., 2018); however, the mortality burden related to cognitive impairment is plausibly underestimated (Stokes et al., 2020). Moreover, a negative correlation between cognitive status and all-cause mortality is particularly noticeable among older adults of advanced age living in lower and middle-income countries (Del Brutto et al., 2024; Duan et al., 2020; Y. Li et al., 2021; Prince et al., 2012). Mild Cognitive Impairment and Interventions Mild cognitive impairment (MCI) is regarded as an intermediate stage between normal aging and dementia (Petersen & Negash, 2008) and recognized as a significant public health concern as a dementia risk (Farias et al., 2009). It is defined as a syndrome of cognitive deterioration beyond what is expected for an individual’s age and educational background, yet it does not significantly interfere with routine daily activities (Petersen et al., 2018; Petersen et al., 1999). Subsequently, various diagnostic criteria and subtypes of MCI have been proposed and modified, alongside the accumulation of evidence supporting validated tools and biomarkers for the pathological characters of MCI (Kasper et al., 2020; Sabbagh et al., 2020). These developments suggest an evolving recognition of MCI as an early stage in the continuum of Alzheimer’s disease (AD) (Bernier et al., 2023; Kasper et al., 2020). According to several community-based prevalence studies in middle income countries, approximately 6% of older adults with MCI progressed to dementia each year in China (Ding et al., 2016), and 23.7% of those with amnestic MCI in Brazil (Lopes et al., 2023). Thus, there is a growing need for early proactive prevention and intervention that can delay the progression of MCI to dementia and also improve the cognitive functions and well-being (Cohen et al., 2021; Lissek & Suchan, 2021). In response to this problem, the effectiveness of pharmacological therapies (e.g., Donepezil, Lecanemab, Galantamine, and Rivastigmine; Dyck et al., 2023; Lai et al., 2020; Zhang et al., 2022) and non-pharmacological interventions (e.g., computerized cognitive training, art and music therapy, physical exercise, and psychoeducational programs; He et al., 2019; Wang et al., 2023; Zuschnegg et al., 2023) have been investigated in older adults with MCI. Nonetheless, it is no clear evident that pharmacological treatments can delay or prevent cognitive decline in older adults with normal cognition and MCI (Fink et al., 2018; Karakaya et al., 2013; Zhang et al., 2022). Meanwhile, previous reviews suggested that nonpharmacological-related multidomain interventions could improve global and higher cognitive functions in older adults with MCI relative to those with single-domain interventions (Bruderer-Hofstetter et al., 2018; Kasper et al., 2020; Salzman et al., 2022). For example, the PRISMA-compliant network meta-analysis by J.-h Liang et al. (2018) suggested that nonpharmacological therapies (i.e., physical exercises and computerized cognitive training) might be more effective than pharmacological therapies in improving cognition and wellbeing. Furthermore, intervention technologies (i.e., immersive virtual reality) capable of delivering multicomponent or multimodal forms (e.g., movement-cognitive interventions) of content and environments may stimulate the recruitment of several cognitive and neural processes and networks (Sherman et al., 2017). Virtual Reality Interventions in Older Adults Virtual reality (VR) has emerged as an economical and valuable approach in clinical contexts (e.g., rehabilitation), allowing for safe and interactive environments to practice cognitive and motor activities (Corregidor-Sánchez et al., 2020). It offers numerous benefits and advantages over traditional cognitive and physical interventions, including increased ecological validity, multi-module flexibility, as well as enhanced motivation and compliance with the interventions. Although VR interventions have proven effective and safe, previous investigations have demonstrated that VR scenarios cannot be considered fully comparable to real-life contexts regarding memory performance enhancement (Ceccato et al., 2024; Monaro et al., 2024). Nonetheless, similar neuro-patterns between VR stimulation and physical environments (i.e., physical downhill skiing) have been observed using EEG power spectrum analysis, with VR and physical environments exhibiting values within the interval of 26.5-32.4 mV, in contrast to the desktop application, which ranged from 10-15.5 mV (Petukhov et al., 2020). The perceived realism of VR, which requires physical mobility and involves higher bioenergy expenditures along with the activation of frontal brain activities, may explain the similarities in brainwaves observed between the virtual and physical worlds (Petukhov et al., 2020). Furthermore, immersive VR experiences using head-mounted display may promote autobiographical retrieval mechanisms compared to conventional on-screen experiences (i.e., a PC condition). Specifically, the VR condition showed lower theta amplitude at the frontal-midline electrode site (i.e., Fz) compared to the PC condition, suggesting a reduced memory load during retrieval in the VR condition. In addition, the reduced alpha amplitude at the occipital electrode site in the VR condition also reflects more effortless memory access relative to the PC condition (Kisker et al., 2021). Thus, to enhance memory performance in VR settings, interventions should necessitate active interaction from participants and manipulate environment factors, namely image quality, emotional engagement, sense of presence, and the congruent object/environmental schemas of the presented stimuli to benefit object recall (Cadet et al., 2022; Koch & Coutanche, 2024). In addition to memory enhancement, VR interventions have been widely utilized to enhance cognitive functions (i.e., processing speed, visual attention, working, spatial and episodic memory, verbal fluency executive functions, and cognitive reserve) and physical performances (i.e., balance and gait) in older adults with both healthy cognition (Gamito et al., 2020; Makmee & Wongupparaj, 2022; Percy et al., 2023; Rendon et al., 2012; Rodríguez-Almagro et al., 2024; Zajc-Lamparska et al., 2019) and MCI (H. Kim et al., 2021; Liao et al., 2020; Thapa et al., 2020). However, a definite conclusion of VR cognitive-based intervention on older adults with MCI cannot be reached in comparison to those of normal cognition (H. Kim et al., 2021). The Present Study Although VR intervention has received considerable attention as a cost-effective mode for administering cognitive and physical interventions, much of the potential remains under-investigated. Many applications to date lack underlying theories for supporting immersive VR methodologies (Carroll et al., 2021) and guidelines for developing quality interventions (Wight et al., 2016). In addition, few studies have explored the effectiveness of immersive VR interventions on the set of high-level cognitive functioning, including verbal/visuospatial short-term memory, executive function, and well-being, in both older adults with and without impairment, as indexed by improved cognitive functions and corresponding changes in brain electrical activities. Apart from neuropsychological parameters, resting-state EEG indices may be a promising candidate as an electrophysiological measure of VR training effectiveness (Thapa et al., 2020; Yang et al., 2022). In resting-state EEG rhythms, increases or decreases in signal power/intensity and latency could reflect resource allocation mechanisms and other neurophysiological changes preceding goal-directed behavior (Buján et al., 2022; Finnigan & Robertson, 2011; Lopez et al., 2024). The EEG signals have been proven to be an effective tool in clinical and non-clinical settings, containing information concerning cognitive, emotional, and physiological processes related to changes in brain electrical activity (Päeske et al., 2023). Resting-state EEG has been employed as a biomarker for MCI and AD diagnosis as well as for the early detection of treatment effects on neuronal functions in older adults with these conditions (Scheijbeler et al., 2023). Specifically, lower efficiency in the frontal theta and alpha bands has been observed in individuals with MCI (S.-E. Kim et al., 2023), and theta and alpha power at frontal electrode sites have been supported as biomarkers for the therapeutic effect of interventions (Trenado et al., 2023). Therefore, the present study aimed to investigate the effects of an immersive VR cognitive-based intervention on higher cognitive functions and electrophysiological changes (i.e., resting-state EEG) in community-dwelling older adults with MCI and normal cognition. Study Design A pretest-posttest control group design was used to investigate the effectiveness of the immersive VR cognitive-based intervention in older adults, both with and without MCI. In this design, participants were allocated to a passive control group (nonintervention) for older adults without MCI and two experimental groups (VR intervention): one for older adults without MCI and another for those with MCI. The dependent variables (i.e., behavioral and EEG parameters) were assessed twice (i.e., pretest and posttest) for three groups. For each participant, morning or afternoon test sessions were matched between pretest and posttest measures. Participants Utilizing the G*power 3 software (Faul et al., 2007) and considering the average effect size from a prior investigation (¦ = 0.36; ) (Makmee & Wongupparaj, 2022), the required sample size for three groups to achieve a statistical power of .80 and a error rate of .05 was determined to be 72 in total. To account for an anticipated attrition rate during the follow-up assessment of the VR intervention (i.e., ~18) (Lin et al., 2018), the current study aimed to recruit a minimum of 30 participants per group, totaling 90 participants. Upon approval by Burapha University – Institutional Review Board (BUU-IRB: HU042/2566), the research teams commented participant recruitment for the study by contacting local senior clubs and posting advertisements on social media platforms such as Facebook and the Line application. Participants in both the control and experimental groups received compensation of USD14 per session to cover their travel expenses and time. The diagnostic criteria for MCI were in line with Petersen et al. (2018), to evaluate: i) the existence of memory problems, a subjective memory complaint questionnaire (SMCQ) (Youn et al., 2009) was utilized; ii) noticeable memory deterioration was measured using the Montreal Cognitive Assessment (MoCA) version 8.1 or 8.2 – Thai translations (www.mocatest.org) (cutoff score £21) (Yeung et al., 2020); and iii) the preservation of typical cognitive function in daily activities was assessed through the Barthel activities of daily living (ADL) index and instrumental ADL index (iADL) (Senanarong et al., 2003). In addition, older adults with depressive symptoms were excluded from the study (Ismail et al., 2017). The 30-item Thai geriatric depression scale (TGDS) was used to detect depression of Thai older adults (Train the brain forum committee, 1994). Interested individuals underwent a set of screening tests (i.e., interview checklists and self-report questionnaires) according to inclusion and exclusion criteria related to hearing, vision, motor and cognitive functions, educational background, as well as history of brain injury and psychiatric disorders. The inclusion criteria for non-MCI older adults included: being aged between 60 and 80 years, having the ability to read, write, and communicate in Thai, no depression (TGDS cutoff score £13) (G. Liang et al., 2009), normal vision or vision corrected to normal with glasses, and no history of brain injuries, surgeries or psychiatric disorders. Exclusion criteria included hearing or visual impairment, or physical (motor) disability that would hinder full participation in intervention sessions. A total of 90 eligible older adults were randomly assigned to one of the three groups using Excel: MCI-experimental (n = 31), non-MCI experimental (n = 29), and non-MCI passive control (n = 30). Research assistants were blinded to participants’ group status, distinguishing between healthy older adults and those with MCI. The Immersive VR Cognitive-based Intervention: Materials The development of the immersive VR cognitive-based intervention was divided into five steps in line with the ADDIE model (Branch, 2009) and six steps in quality intervention development (6SQuID) (Wight et al., 2016). First, the existing literature on cognitive decline in older adults with MCI and normal cognition was assessed and synthesized concerning the causes, treatment, tests/instruments, and contextual factors. Target variables for intervention, including processing speed, short-term memory (STM), executive function (EF), and well-being, were identified and characterized as a theoretical framework of the immersive VR training program. Moreover, the theories of interactive cognitive complexity (Tennyson & Breuer, 1997) and dual-channel processing (Mayer & Moreno, 2003) were utilized to enhance cognitive and affective engagement and facilitate executive functioning systems by employing verbal and visuospatial stimuli. Second, various VR software and hardware options were compared and selected. The mechanisms of change and delivery were analyzed, along with the design of immersive VR environments and models. The underlying mechanisms of cognitive enhancement in the experimental groups involved reliance on improving the working memory (WM), visuomotor adaptation (N. Li et al., 2021; N, Li et al., 2023; Makmee & Wongupparaj, 2022) and perceived entertainment of VR applications of older adults (Alarcon-Urbistondo et al., 2024). Next, content of the interventions, including learning outcomes, tasks, feedback stimulation (H. Kim et al., 2022) and outputs (i.e., response accuracy and time) were delineated. The VR scenes, objects, main activities and total session length (in minutes) were summarized in Table 1 and Figure 1A. Figure 1 Screenshots of the Kitchen, Bedroom, Garden, Bbathroom, and Living/Working Room, respectively (A) and the Participant Wearing the VR Headset and Controlling the Wireless Joysticks (B), which Was Administered via iPad (C).   Third, the immersive VR prototype underwent pilot testing and evaluation with five older adults resembling the target population. The feasibility and positive acceptability of the intervention were largely confirmed, with low and manageable levels of motion sickness reported. The common symptoms from an older adult included dizziness and sweating, which were induced during an initial session of the immersive VR training. Furthermore, the content validity index (CVI) and a focus group were employed to assess the content validity of the developed immersive VR intervention, involving three experts (Yaghmaie, 2003) in gerontology and geriatric medicine, neuroscience, and VR technology. The average CVI score or the scale-level CVI was 1.00, indicating strong evidence of content validity (Polit & Beck, 2006). Finally, the training protocols and procedures were developed and administered. The device used for the training was the Oculus Quest 2 HMD (head-mounted display) with two wireless joystick controllers. It is a standalone unit equipped with a stereoscopic display and all the necessary components to deliver VR experiences with a refresh rate of 72 Hz, a resolution of 1832 x 1920 per eye, and a field of view of 89 degrees. The immersive VR contents were created by using Unity3D game engine and administered using an iPad 9th generation with 10.2-inch display running iPadOS 15, equipped with a hexa-core CPU, as depicted in Figure 1 B and C. The USB-C 3.0 connectivity controllers were used to perform tasks such as selecting, grabbing, relocating, and arranging animated objects within virtual environments. The Immersive VR Cognitive-based Intervention: Procedures The immersive VR cognitive-based intervention was delivered in sessions lasting 60 minutes each, conducted twice a week over four consecutive weeks, amounting to a total of eight sessions. Each session consists of four stages: (i) selecting a character from among 14 senior men and women with various careers in a reception room; (ii) choosing a VR scene from five environments (i.e., kitchen, bedroom, garden, bathroom, and living/working room); (iii) performing tasks or activities in accordance with step-by-step instructions; and (iv) receiving feedback and recording the score and time used in the system (see Figure 2 A, B, and C). During the one-hour VR training, with one environment per session, each session consisted of four main activities in a step-by-step order. The sequence of activities was arranged from basic to complex tasks, requiring increasing cognitive control memory retrieval, and cognitive load. Once the participants completed all five environments, they were allowed to choose and replay any environment until the end of the final session. The VR training system provided feedback to participants after each activity and at the end of each session. Specifically, when participants successfully completed a task, a message appeared at the bottom of the VR scenes, indicating that the mission had been accomplished. Additionally, upon completing each session, a message appeared at the bottom of the final VR sciences for each environment, displaying the score (i.e., one to five stars) and the time spent on that session. The motion sickness of participants was assessed by an interview for three groups. Thirteen older adults without cognitive impairment exhibited symptoms including dizziness, sweating, and vomiting; however, these symptoms appeared only on the first day of the training and disappeared thereafter. Nineteen older adults with MCI experienced motion sickness on their first day of training. Research assistants recorded all symptoms reported by the participants. If any symptoms occurred, the research assistants advised the participants to rest, or participants could request a short break on their own. Furthermore, the research assistants conducted regular checked-ins with participants every 10 minutes to ensure the absence of any symptoms. Figure 2 The VR Scenes at Reception for Participant Character (A) and Virtual Room (B) Selection, the Beginning and End of Tasks with Feedback (C), and EEG Recordings during both Eye-open and Eye-closed Conditions.  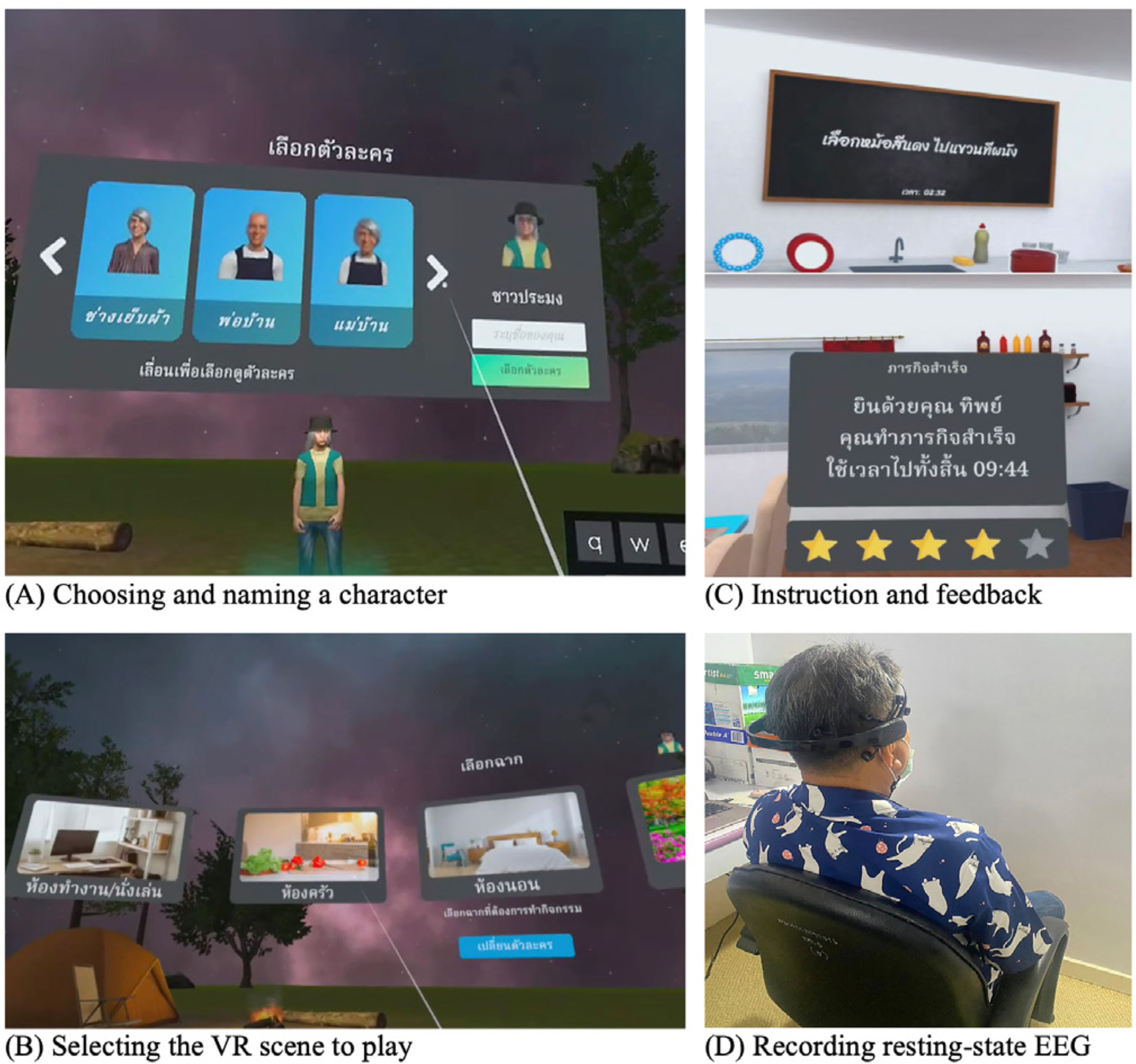 Measures Three types of tests were performed to assess STM (i.e., verbal and visuospatial), EFs (i.e., inhibition, updating, and shifting), well-being, and resting-state EEG of older adults. All participants completed all tests in the laboratory at the Center of Excellence in Cognitive Science (CECoS) during both the pre- and post-intervention sessions, which were approximately 30 days apart. STMs Verbal STM. The forward digit span test was employed to index the verbal STM of participants (Richardson, 2007; Wongupparaj et al., 2017). Verbal STM was evaluated using a computerized version of the forward digit span included in the Psychology Experiment Building Language (PEBL) test battery version 2.1 (Mueller & Piper, 2014). A string of numbers was displayed on a 19” LCD computer screen, and participants were instructed to repeat them in the same sequence. The shortest list length consists of three digits, while the longest list comprises up to ten digits. Each length is composed of two consecutive trials. One point was awarded for each correct trial, resulting in total scores ranging from 1 to 16 points. The Inter-Stimulus Interval (ISI) was set to 1,000 milliseconds (ms.), and the inter-trial interval (ITI) was set to 1,500 milliseconds. The test-retest reliability coefficient was found to be .62 (p < .01, 95% bootstrap confidence interval = .48-.74). Visuospatial STM. The forward Corsi-block tapping test was used to assess the visuospatial STM of older adults participating in the current study (Kessels et al., 2000; Wongupparaj et al., 2017). A computerized version of the Forward Corsi-Block Tapping Test was administered using PEBL (version 2.1) test battery (Mueller & Piper, 2014). During the test, nine square blocks were displayed on the 19-inch computer monitor. The three parameters of the test setup (i.e., positioning, block quantity and sequence order) combine to create a visuospatial path (Arce & McMullen, 2021). Participants were required to remember the sequence of lighting up the blocks one after another and then replicate this sequence by clicking the corresponding blocks on the computer screen using a computer mouse. The sequence involved lighting up blocks ranging from two to nine, with each sequence comprising two consecutive trials. At the end of the test, the total score was calculated as the product of the block span and the number of correct trials (Hazarika & Dasgupta, 2020), resulting in scores ranging from 0 to 162. The ISI and ITI were both set to 1,000 ms. The test-retest reliability coefficient was found to be .81 (p < .01, 95% bootstrap confidence interval = .71-.89). EFs Inhibition. Go/nogo test was employed to assess response inhibition or inhibitory control, which is a principal component of EFs (Diamond, 2013; Littman & Takács, 2017). A computerized version of the go/nogo was administered using PEBL (version 2.1) test battery (Mueller & Piper, 2014). During the test, participants observed a sequence of letters (P or R) on the 19-inch computer screen and pressed a button on a keyboard in response to target letters. Each letter (P or R) was displayed within a 2 x 2 grid along with four stars, shown for 500 milliseconds with ISI of 1,500 ms. The go/nogo test comprised two conditions: the P-Go condition, involving 160 trials, and the R-Go condition, also with 160 trials. In the P-Go condition, participants were instructed to press the right shift key when the target letter P appeared and to refrain from responding to the non-target letter R. The ratio of target to nontarget letters was 80:20. Subsequently, participants completed the R-Go condition, responding to the target letter R and withholding responses to P. The trial count and ratio were the same as in the P-Go condition. Overall, the test included 320 trials, with mean response accuracy (RA) as a primary measure, ranging from 0.00 to 1.00. The test-retest reliability coefficient was found to be .33 (p < .01, 95% bootstrap confidence interval = .13-.53). Updating. The backward digit span test was utilized to measure the updating function of WM, which is a core function of EFs (Diamond, 2013; Makmee & Wongupparaj, 2022; Wongupparaj et al., 2017). A computerized version of the backward digit span test was administered using PEBL (version 2.1) test battery (Mueller & Piper, 2014). During the test, a string of numbers was displayed on a 19-inch computer screen. Participants were instructed to repeat these numbers in reverse order, with sequences ranging from three to ten digits in length. Each sequence consisted of two consecutive trials. One point was awarded for each correct trial, resulting in total scores ranging from 1 to 16 points. The ISI was set to 1,000 ms, while the ITI was set to 1,500 ms. The test-retest reliability coefficient was found to be .73 (p < .01, 95% Bootstrap confidence interval = .60-.84). Shifting. The connections test is a variant of trail-making task with minimal motor demands (Mueller & Piper, 2014; Salthouse et al., 2000). The connections test was used to test cognitive shifting or task switching of participants (Salthouse et al., 2000). A computerized version of the connections test was administered using PEBL (version 2.1) test battery (Mueller & Piper, 2014), with stimuli translated into Thai letters. During the test, participants were required to connect a set of circles that are labeled with letters and/or numbers and appeared on a 7 x 7 grid. Each trial composed of a sequence of letters (i.e., ), numbers (i.e., 1-2-3-4), or an alternative sequence (i.e., 1--2-) with a time limit to complete at 20 seconds. Target switching score was calculated and used as a primary measure (Salthouse, 2011). The test-retest reliability coefficient was found to be .59 (p < .01, 95% bootstrap confidence interval = .39-.74). Well-being The Well-being of Older People (WOOP) (Hackert et al., 2020) was employed to assess key domains of well-being in older adults, focusing on their functioning rather than their capabilities. The WOOP measure contains nine items and was translated into Thai version in line with guidelines for establishing cultural equivalency and translation of instruments (Beaton et al., 2000). The translation process involved two bilingual professional translators who conducted forward-backward translation. For all items, response options were defined on a 5-point Likert scale, ranging from 1 (bad) to 5 (excellent), reflecting levels of well-being-relevant functioning. The total score ranged from 9 to 45, with higher scores indicating higher levels of well-being among older participants. The reliability coefficient via Cronbach’s alpha was .71. EEG Recording, Data Acquisition, and Pre-processing Resting-state EEG recording from three groups of participants was used to represent changed cognitive functions and neuronal organization (Buján et al., 2022; Rogala et al., 2020) and index neurophysiological makers of intervention effectiveness (Trenado et al., 2023). In addition, several studies have suggested that resting-state EEG potentially reflected cognitive declines in older adults (Kopanová et al., 2024), while theta-gamma ratio (TGR) at frontal regions correlated with STM capacity (Kamiski et al., 2011), an increase in relative theta power (RTP) at frontal regions represented enhanced EFs (Finnigan & Robertson, 2011), and alpha asymmetry (AA) index at frontal regions correlated with wellbeing (Cannard et al., 2021; Wutzl et al., 2023). During the resting-state EEG recording, participants were instructed to sit comfortably on a chair and gaze at a white wall for 2.5 minutes with their eyes open, followed by 2.5 minutes of keeping their eyes closed (see Figure 2 D). The duration of pre-and-post EEG recording was approximately three to four weeks. Raw EEG was recorded using a 14-channel wireless EMOTIV® EPOC X headset on the 10-20 system. The 14 active electrodes include frontal regions (i.e., AF3, AF4, F3, F4, FC5, FC6, F7, and F8), temporal regions (i.e., T7 and T8), parietal regions (i.e., P7 and P8), and occipital regions (i.e., O1 and O2), along with two reference electrodes positioned at CMS and DRL. The sampling rate of the headset was set to 128 Hz with 16 bits (0.1275 mV) voltage resolution. The bandwidth of the recording was 0.16 to 43 Hz along with digital notch filters at 50 and 60 Hz. Impedance at each electrode was reduced by applying a saline solution until the impedance levels reached the required threshold specified by the EMOTIV PRO software. EEG signals were preprocessed to remove artifacts (i.e., line noise, cardiac field, ocular and muscle movement artifacts) using plugins of EEGLAB v2024.0, a MATLAB v2023b toolbox. The preprocessing pipeline consisted of several steps: offline referencing to a common average reference, offline filtering from 1 to 43 Hz, baseline correction, and Independent Component Analysis (ICA) label algorithm (i.e., ICLable v1.6) to remove EEG artifacts (Delorme & Makeig, 2004). Subsequently, the continuous EEG data were segmented into non-overlapping epochs of 2 seconds using a Hanning window (10% window length). The segmented EEG epochs were then processed to obtain absolute power (in mV2/Hz) using a Fast Fourier transformation (FFT) for both eyes-open and eyes-closed conditions. These power values were subsequently averaged across five frequency bands: delta (1-3 Hz), theta (4-7 Hz), alpha (8-12 Hz), beta (13-30 Hz), and gamma (31-43 Hz). The relative EEG power for each band was calculated by dividing a selected absolute bandpower by all of the five frequency bands within the 1-30 Hz range. Data Analysis To test the efficacy of the immersive VR cognitive-based intervention, descriptive (i.e., percentage, mean, standard deviation, skewness, and kurtosis) and inferential statistics (i.e., repeated-measures ANOVA and MANOVA) were employed. In addition, repeated-measures ANOVA tests, followed by Bonferroni post hoc tests, were conducted to control type I errors. Furthermore, partial omega squared ( In addition, repeated-measures ANOVA tests followed by Bonferroni post hoc test were conducted. The AA index was calculated for each participant using the equation (Thompson & Ong, 2018): Where F4 (alpha) and F3(alpha) represent absolute alpha (8-12 Hz) power values at the right and the left frontal electrode sites, respectively and ln stands for natural log. All analyses were performed using IBM SPSS Statistics, version 29.0 (SPSS Inc., Chicago, Illinois, USA). Participant Characteristics Ninety older adults who met the inclusion criteria and passed the screening tests for healthy or MCI conditions were divided into three groups: the MCI experimental (n = 31), the non-MCI experimental (n = 29), and the non-MCI passive control (n = 30) groups. For the MCI experimental group, the mean age and years of education were 68.19 ± 5.03 years (min-max: 60-79) and 10.55 ± 4.66 years (range: 6-19), respectively. All participants were Thai nationals, with 80.6% being female, 54.8% married, and 64.5% identifying as housewives and/or merchants. The mean scores of SMCQ, ADL, iADL and TGDS were 6.36 ± 3.59 (min-max: 0-13), 19.81 ± 0.48 (min-max: 18-20), 9.03 ± 0.31 (min-max: 8-10) and 12.00 ± 1.29 (min-max: 9-13), respectively. For the non-MCI experimental group, the mean age and years of education were 66.31 ± 3.12 years (min-max: 61-74) and 13.11 ± 4.53 years (range: 6-19), respectively. All participants were Thai nationals, with 86.2% being female, 44.8% married, and 62% identifying as housewives and/or retired employees. The mean scores of SMCQ, ADL, iADL and TGDS were 4.38 ± 3.11 (min-max: 0-10), 19.90 ± 0.31 (min-max: 19-20), 9.00 ± 0 (min-max: 9), and 11.03 ± 1.68 (min-max: 8-13), respectively. For the non-MCI passive control group, the mean age and years of education were 64.97 ± 3.35 years (min-max: 60-75) and 13.40 ± 4.05 years (range: 6-19), respectively. All participants were Thai nationals, with 86.7% being female, 46.7% married, and 53.3% identifying as housewives. The mean scores for SMCQ, ADL, iADL, and TGDS were 3.00 ± 2.59 (min-max: 0-10), 19.93 ± 0.25 (min-max: 19-20), 8.96 ± 0.18 (min-max: 8-9), and 11.10 ± 1.40 (min-max: 8-13), respectively. Only mean ages of the three groups were significantly different, and this variable was used as a covariate in ANOVA/MANOVA. No missing data was found for the current analysis. The non-normally distributed data were transformed by using natural log to mitigate issues of kurtosis and skewness. Behavioral Findings Table 2 indicates the enhanced scores of verbal STM in experimental groups: MCI, t(30) = 2.09, p < .05, and non-MCI, t(28) = 2.76, p < .01, conditions. However, no difference between pretest and posttest scores was observed in the non-MCI control group, t(29) = 0.64, p >.05. Moreover, the posttest scores were significantly different among the three groups, F(2, 87) = 3.30, p <. 05, but the effect size was small (w2 = .05). Post hoc comparisons using the Bonferroni correction indicated that the posttest scores of verbal STM in the MCI experimental group were significantly lower than those in the non-MCI experimental and control groups (mean differences = -0.82 and -0.83, p = .03 and .03, respectively). Nonetheless, the posttest scores of verbal STM of the non-MCI experimental group did not differ from those in the control group (mean difference = -0.01, p = .98) (see Table 4). Table 2 Behavioral Outcomes of the Control and Experimental Groups for STMs, EFs, and Well-being  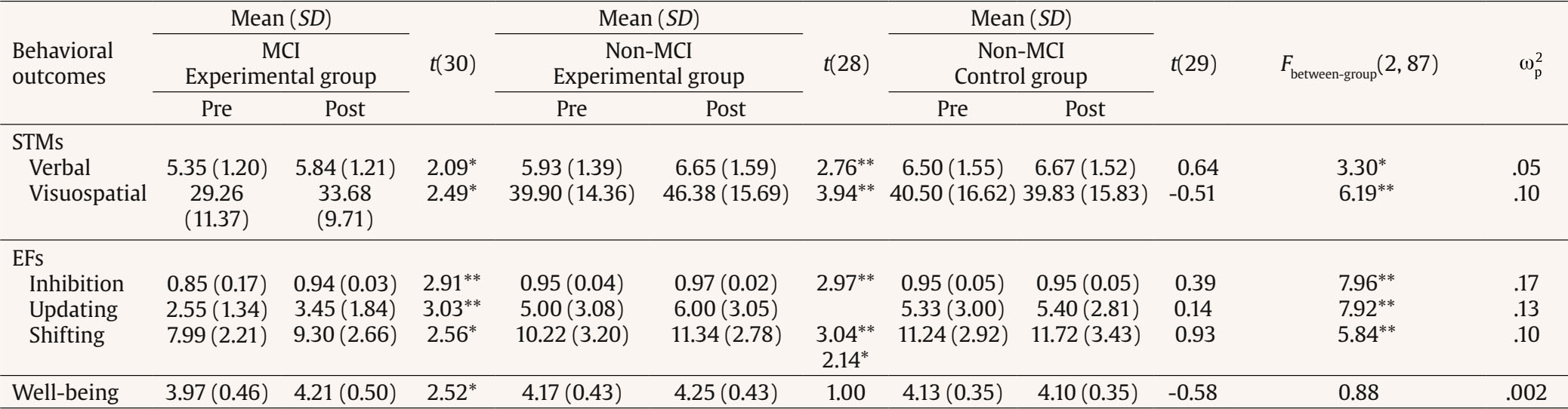 Note. Age as a covariate of the analysis. *p <. 05, **p < .01. Table 3 Resting-state EEG Outcomes of the Control and Experimental Groups for STMs, EFs, and Well-being  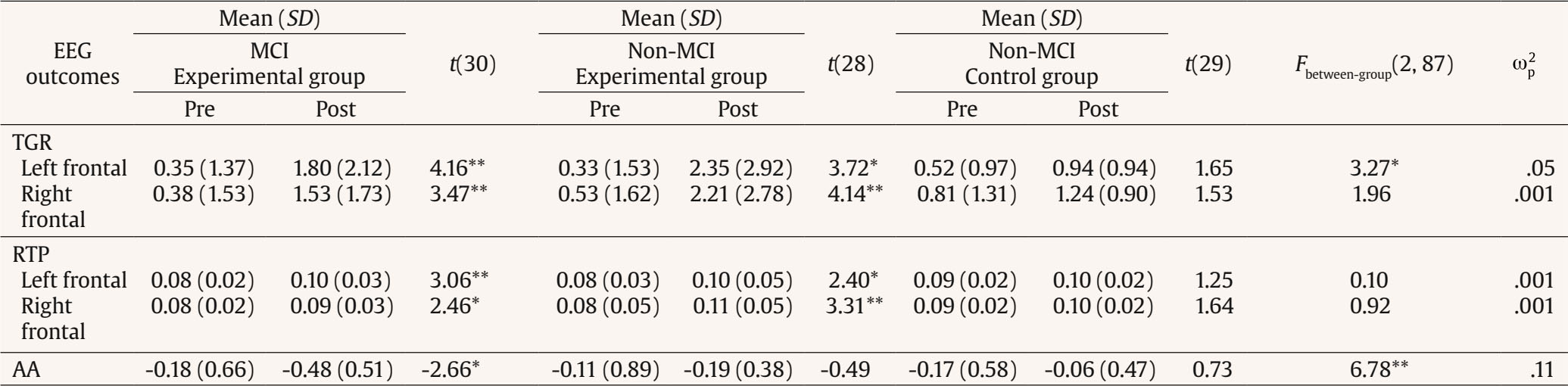 Note. Age as a covariate of the analysis. *p <. 05, **p < .01. Table 4 Paired t-test Comparisons for Posttests of Behavioral Outcomes among Three Groups  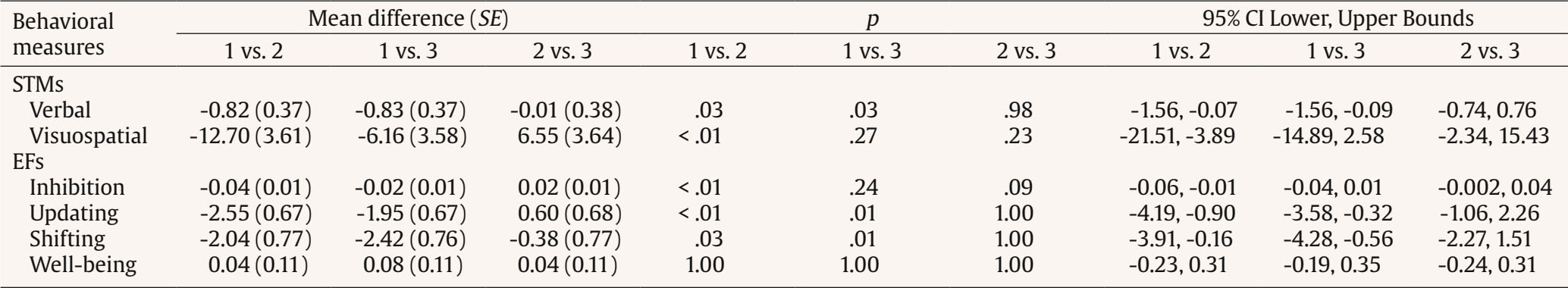 Note. 1 = MCI experimental group, 2 = non-MCI experimental group, and 3 = non-MCI control group. Similarly, the posttest scores of visuospatial STM were significantly greater than the pretest scores in experimental groups, tMCI(30) = 2.49, p < .05 and tnon-MCI(28) = 3.94, p < .01, respectively; nonetheless, this observation was not found in the control group, t(29) = -0.51, p > .05). Furthermore, the posttest scores among the three groups significantly differed, F(2, 87) = 5.20, p < .01, with the effect size indicating a moderate magnitude (= .10). Post-hoc comparisons using the Bonferroni correction indicated that the posttest scores of visuospatial STM in the MCI experimental group were significantly lower than those in the non-MCI experimental group (mean difference = -12.70, p < .01). However, the posttest scores of visuospatial STM did not differ between the MCI experimental and the control groups, nor between the non-MCI experimental and control groups (mean differences = -6.16 and 6.55, p = .27 and .23, respectively) (see Table 4). For EFs, all three parameters showed significant gains only in experimental groups: MCI, tinhibition(30) = 2.91, p < .01; tupdating(30) = 3.03, p <. 01 and tshifting(30) = 2.56, p < .05, and non-MCI conditions, tinhibition(30) = 2.97, p < .01; tupdating(30) = 3.04, p < .01 and tshifting(30) = 2.14, p < .05. Additionally, the posttest scores for all EF parameters differed significantly among the three group, Finhibition(2, 87) = 7.96; p <. 01, Fupdating(2, 87) = 57.92 p < .01; and Fshifting(2, 87) = 5.84, p < .01), with effect sizes ranging from moderate to large ( = .10-.17). Post hoc comparisons using the Bonferroni correction indicated that the posttest inhibition scores of the MCI experimental group were significantly lower than those the non-MCI experimental group (mean differences = -0.04, p < .01). However, the posttest inhibition scores did not differ between the MCI experimental and control groups, nor between the non-MCI experimental and control groups (mean differences = -0.02 and 0.02, p = .24 and .09, respectively) (see Table 4). Similarly, post hoc comparisons using the Bonferroni correction indicated that the posttest updating and shifting scores in the MCI experimental group were significantly lower than those in the non-MCI experimental and control groups (mean differences for updating = -2.55 and -1.95, p < .01 and =.01 and mean differences for shifting = -2.04 and -2.42, p = .03 and .01, respectively). However, the posttest updating and shifting scores of the non-MCI experimental group did not differ from those in the control group (mean differences = 0.60 and -0.38, ps = 1.00) (see Table 4). Finally, the significant difference between the pretest and the posttest score of well-being was only observed in the MCI experimental group, t(30) = 2.52, p < .05. No improvement of well-being was observed in the non-MCI experimental and control group, tnon-MCI experimental (28) = 1.00, p > .05 and tnon-MCI control (29) = -0.58, p > .05, respectively). Furthermore, there was no difference in the posttest scores of well-being among the three groups, F(2, 87) = 0.88, p > .05. EEG Findings Table 3 shows significantly higher TGR values in the posttest condition relative to the pretest condition of the experimental groups at the left frontal electrode site (i.e., AF3), tMCI(30) = 4.16, p < .01 and tnon-MCI(28) = 3.72, p < .05, respectively. No gain score was observed for the posttest condition in the control group, t(29) = 1.65, p > .05. Furthermore, the posttest scores were significantly different among the three groups, F(2, 87) = 3.27, p < .05, but the effect size was small (= .05). Post hoc comparisons using the Bonferroni correction indicated that the posttest TGR values in the non-MCI experimental group were significantly higher than those in the non-MCI control group (mean difference = 1.41, p = .04). However, the posttest TGR values did not differ between the MCI and the non-MCI experimental groups, nor between the MCI experimental and the non-MCI control groups (mean differences = -0.56 and 0.85, p = .96 and .38, respectively) (see Table 5). Table 5 Paired t-test Comparisons for Posttests of EEG Outcomes among Three Groups  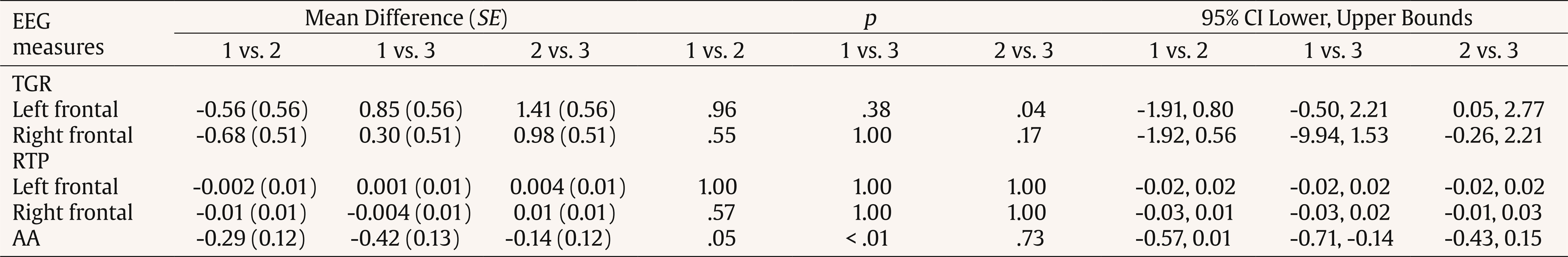 Note. 1 = MCI experimental group, 2 = non-MCI experimental group, and 3 = non-MCI control group. Significant gain scores of the TGR posttest were only observed at the right frontal electrode site (i.e., AF4) in the experimental groups, tMCI(30) = 3.47, p < .01 and tnon-MCI(28) = 4.14, p < .01, respectively. However, no difference between pretest and posttest TGR values was observed in the control group, t(29) = 1.53, p > .05, nor were differences observed among the posttest TGR values among the three groups, F(2,8 7) = 1.53, p > .05. Similar trends to those observed in TGR values were also found in RTP values. Specifically, the higher RTP values of the posttest condition relative to the pretest condition were only observed in the experimental groups at both left, tMCI(30) = 3.06, p < .01 and tnon-MCI(28) = 2.40, p < .05, respectively) and right, tMCI(30) = 2.46, p < .05 and tnon-MCI(28) = 3.31, p < .01, respectively frontal electrode sites (i.e., F3 and F4). Nonetheless, no differences were observed among the posttest RTP values among the three groups for both left, F(2, 87) = 0.10, p > .05, and right, F(2, 87) = 0.92, p > .05, frontal electrode sites. Finally, a significant difference between pretest and posttest AA values was observed only in the MCI experimental group, t(30) = -2.66, p < .05. Furthermore, the posttest AA values among the three groups significantly differed, F(2, 87) = 6.78, p < .01, and the effect size indicated a moderate magnitude (= .11). Post hoc comparisons using the Bonferroni correction indicated that the posttest AA values in the MCI experimental group were significantly lower than those in the non-MCI control group (mean difference = -0.42, p < .01). However, the posttest AA values did not differ between the MCI and the non-MCI experimental groups, nor between the non-MCI experimental and control groups (mean differences = -0.29 and -0.14, p =.05 and .73, respectively) (see Table 5). The primary objective of the current investigation was to assess the efficacy of immersive VR-based cognitive intervention on verbal and visuospatial STM, EFs and well-being across three groups of community-dwelling older adults, both with and without MCI. The VR intervention was developed in accordance with the ADDIE model and the 6SQuID framework. Verbal and visual content, feedback, as well as interaction were employed to stimulate participants both affectively and cognitively, according to interactive cognitive complexity and dual-channel theories (Mayer & Moreno, 2003; Tennyson & Breuer, 1997). Behavioral measures including verbal and visuospatial STM, inhibition, updating, shifting, and well-being, along with EEG parameters including TGR, RTP, and AA, were used to reflect the effectiveness and the underlying neurophysiological changes of the VR cognitive-based intervention in the MCI experimental (n = 29), non-MCI experimental (n = 31), and non-MCI control (n = 30) groups. In general, the behavioral outcomes supported the effectiveness of the VR cognitive-based intervention in enhancing STMs and EFs among older adults in experimental groups. However, the significant improvement in well-being was particularly observed among older adults with MCI. In relation to verbal and visuospatial STMs, the developed intervention exhibited stronger effects on both types of STM in non-MCI older adults compared to those with MCI. Specifically, the improvement in visuospatial STM was more pronounced than in verbal STM for both older adults with and without MCI. These findings were in line with the principles of interactive cognitive complexity and dual-channel theories (Mayer & Moreno, 2003; Tennyson & Breuer, 1997), suggesting that the developed intervention could activate the dual codes, thereby enhancing the speed of cognitive processing and reducing cognitive load (Mayer & Moreno, 2003). Moreover, older participants were actively engaged in exploring and navigating through various scenes and tasks within virtual environments. These activities may not only stimulate procedural memory but also activate motor brain areas (Jonson et al., 2021), in addition to the regions supporting STM. While the extent of the benefit of the VR intervention on visuospatial STM enhancement remains inconclusive (H. Kim et al., 2022; Plechatá et al., 2021), the present results further support the efficacy of the VR intervention for improving visuospatial STM in both healthy and clinical populations. In addition, participants showed improvement in their scores on verbal STM. This finding is consistent with prior study in that the VR intervention could enhance verbal STM, as demonstrated by improved word recall (Boller et al., 2021). Although the previous literature suggests that the VR intervention could enhance scores of the forward digit span test in older adults with cognitive impairment following stroke (B. R. Kim et al., 2011), the present findings suggest that these benefits may extend to older adults both with and without cognitive impairment. In terms of EF enhancement, the current findings support the existing literature on the enhancement of EFs through VR cognitive-based interventions in healthy (Makmee & Wongupparaj, 2022) and MCI populations (Yu et al., 2023). Moreover, it is also evident from the current study that comparable and positive effects were observed between pre-and-post conditions in both older adults with and without MCI, particularly inhibitory and updating abilities, as evidenced by large effect sizes. It is plausible that the VR cognitive-based intervention could stimulate executive control network of both groups (Liu et al., 2021). The underlying mechanism of EF enhancement may stem from stimulating cognitive (i.e., working memory and visuomotor adaptation) and affective (i.e., positive emotion) components of the VR cognitive-based intervention (Mancuso et al., 2023; Tortora et al., 2024). Thus, it is plausible that the VR cognitive based intervention may enhance WM capacity of older adults and improve visual and motor adaptation (Vandevoorde & Xivry, 2020), thereby enabling older adults to have sufficient cognitive and affective resources to control attention, thought, emotion, and behavior (Diamond, 2013). In addition, it is evident that the VR intervention yielded positive cognitive performances due to high adherence rates of older adults (Ortiz-Mallasén et al., 2024), which was also pronounced in the current investigation. In relation to well-being, the current findings partially supported the hypothesis. In relation to well-being, the current findings partially supported the hypothesis. The improved well-being score was only evident in the MCI experimental group, implying that the VR cognitive-based intervention might confer greater benefits to individuals with MCI relative to non-MCI older adults. This finding was consistent with previous pilot studies (Baragash et al., 2022; Chaze et al., 2022; Dermody et al., 2020; Restout et al., 2023), suggesting positive effects of VR on physical, cognitive, and emotional well-being in both older adults with and without MCI. Nonetheless, the effectiveness of the intervention in promoting well-being may be related to the VR components (i.e., content, environments, and level of immersion) that cater to the skill acquisition and mastery of community-dwelling older adults with MCI. These VR components may facilitate cognitive and affective engagement in this group. Apart from the efficiency of the VR cognitive-based intervention in the current study, as reflected by behavioral outcomes, resting-state EEG also supported the positive changes in the experimental groups after receiving the VR cognitive-based intervention, as evidenced by the TGR, RTP, and AA indices. First, TGR at frontal electrode sites was derived from the ratio of theta and gamma EEG absolute power and employed to index STMs (Kamiski et al., 2011). According to Lisman (2005), STM capacity depends on the number of gamma cycles that can fit within one theta cycle. The ratio between theta and gamma cycle lengths were found to positively correlate with STM capacity (Pahor & Jaušovec, 2015; Vosskuhl et al., 2015). The developed intervention’s effect possibly resulted in an increase in the TGR, indicating successful maintenance and sequential search of STM representations within the frontal regions for theta rhythm, as well as a more efficient memory load for gamma rhythm (Kamiski et al., 2011) in older adult with and without MCI. Second, a higher power of the resting-state RTP value in the frontal regions was found to correlate with better EFs and was considered a marker of healthy neurocognitive aging in older adults without MCI (Finnigan & Robertson, 2011). The current findings not only support the enhanced RTP values in healthy older adults, but also in older adults with MCI. These findings were consistent with the prior investigations that demonstrated lower theta power in older adults with cognitive declines (Cummins et al., 2008; Cummins & Finnigan, 2007) or physiological aging (i.e., neuronal loss in hippocampus or reduced hippocampal volume) (Vlahou et al., 2014). It is plausible that the elevated RTP values observed in the experimental groups suggest a more efficient updating of working memory, which is related to executive function components (Cummins et al., 2008). Nevertheless, EF has been considered a complex construct (Diamond, 2013), and it is likely that resting-state EEG may not effectively reflect active EF mechanisms in comparison to task-specific midline theta (Cummins & Finnigan, 2007). Furthermore, achieving a larger intervention effect may require sustained cognitive effort and longer-term VR training to enhance the neuroplasticity of the aging brain (Park & Bischof, 2013). Finally, frontal AA was utilized as a measure to assess wellbeing in older adults. Based on the current results, wellbeing encompasses cognitive and affective dimensions. It is assumed that through engagement in pleasurable experiences of VR and enhancement of STMs and EFs, higher levels of wellbeing can be attained in the experimental groups. The more negative AA observed among older adults with MCI after receiving the intervention suggested a left dominance of alpha power. Thus, it is in line with numerous studies have demonstrated greater right frontal AA in the resting state in individuals with depression relative to those without depression (Xie et al., 2023), decreased levels during psychosocial stress (Vanhollebeke et al., 2022), and greater left frontal AA and increased levels of subjective wellbeing (Xu et al., 2018). Moreover, it is also possible to establish a connection between EFs and well-being. According to the asymmetric inhibition model (Grimshaw & Carmel, 2014), inhibitory control possibly plays a significant role in both the left and right frontal cortex. Specifically, asymmetries in the left frontal cortex may inhibit negative distractors, while the right frontal cortex may inhibit positive distractors. Therefore, the VR cognitive-based intervention could stimulate a stronger left frontal mechanism and lead to enhanced well-being in older adults with MCI. The strengths of the current investigation lie in its well-designed VR intervention, which adheres to the ADDIE model and the 6SQuID framework (Branch, 2009; Wight et al., 2016). Additionally, to enhance engagement and stimulate higher cognitive functions in older adults, the intervention incorporates interactive cognitive complexity and dual-channel processing theories. Furthermore, the developed VR cognitive-based intervention was tested on both community-dwelling older adults with MCI and those with normal cognition, ensuring that the present findings are applicable to both clinical and non-clinical populations. The current study incorporated standardized cognitive and electrophysiological markers, enabling the observation of changes in neural activities and behavioral outcomes resulting from the intervention. Nonetheless, there are several limitations that should be taken into consideration. First, the participant characteristics may restrict the generalizability of the study due to a larger proportion of female participants (i.e., > 80%) in both control and experimental groups. Second, the intervention consists of eight sessions, and its immediate changes were observed via computerized tests and EEG signals; however, follow-up was not conducted to assess the durability or retention of the treatment effects. Third, the VR training system does not offer real-time difficulty adjustments based on the performance of older adults, which may limit its effectiveness in targeting and improving specific cognitive abilities. Fourth, it is plausible that a ceiling effect may have influenced the go/nogo test. Future studies should consider using an adaptive test to adjust difficulty levels from low to high (Forero et al., 2023). Fifth, it is worth noting that the behavioral and neurophysiological outcomes observed in this study might primarily represent near-transfer effects. Considering the multidimensional construct of current markers, it is imperative to incorporate behavioral indicators of STMs, EFs, and wellbeing in daily life to enhance the interventions’ effectiveness in achieving far-transfer effects (Zelinski, 2009). Finally, the current investigation lacks an active control group, which may limit causal inference and reduce the generalizability of the findings. An immersive VR cognitive-based intervention was developed and assessed in a sample of 90 older adults, including individuals with MCI and those with normal cognition. Specifically, the sample consisted of 31 MCI participants in the experimental group, 29 participants with non-MCI in the experimental group, and 30 non-MCI participants in the passive control group. Significant gain scores were observed in behavioral and electrophysiological indices associated with verbal and visuospatial STMs, EFs – namely inhibition, updating, and shifting – as well as wellbeing within the experimental groups. The current investigation offers intensive protocol development of VR training and incorporate behavioral cognitive and EEG outcomes to reflect multidimensional aspects of intervention effectiveness in older adults with and without MCI. However, the VR cognitive-based intervention demonstrated efficacy specifically in enhancing the wellbeing of older adults with MCI. Subsequent research may necessitate a follow-up period to explore the long-term effects of the intervention. Conflict of Interest The authors of this article declare no conflict of interest. Cite this article as: Makmee, P. Wongupparaj, P. (2025). VR cognitive-based intervention for enhancing cognitive functions and well-being in older adults with mild cognitive impairment: Behavioral and EEG evidence. Psychosocial Intervention, 34(1), 37-51. https://doi.org/10.5093/pi2025a4 Funding This research is funded by the National Research Council of Thailand (NRCT N32A660118), and it is part of the research project entitled ‘Integrated virtual reality application development for enhancing executive functions, memory, and well-being in healthy older adults and older adults with mild cognitive impairment: behavioral and brainwave study’. |
Cite this article as: Makmee, P. & Wongupparaj, P. (2025). VR Cognitive-based Intervention for Enhancing Cognitive Functions and Well-being in Older Adults with Mild Cognitive Impairment: Behavioral and EEG Evidence. Psychosocial Intervention, 34(1), 37 - 51. https://doi.org/10.5093/pi2025a4
Correspondence: peera.wo@go.buu.ac.th (P. Wongupparaj).Copyright © 2026. Colegio Oficial de la Psicología de Madrid








 e-PUB
e-PUB CrossRef
CrossRef JATS
JATS






